
Antioxidant BHT 264
CAS:128-37-0
Purity:99%
Contact Now
We will contact you as soon as possible
Your Location:Home >Products >Fine Chemicals >19715-19-6




Product Details
|
Chemical Properties |
White powder |
|
Uses |
3,5-di-tert-butylsalicylic acid is an important substance widely used as synthetic materials such as pressure sensitive recording paper, agriculture chemicals, antioxidants and so on. 3,5-di-tert-butylsalicylic acid and its derivatives, both metal salts and complexes, possess interesting triboelectrical properties. They are frequently used as charge control additives in dry xerographic toner at low concentrations to control the charging characteristic of toners. |
InChI:InChI=1/C15H22O4/c1-14(2,3)9-7-8(13(18)19)11(16)10(12(9)17)15(4,5)6/h7,16-17H,1-6H3,(H,18,19)
The initial yield of 3,5-di-t-butylsalic...
Finally, DIANANE (3) was condensed with 2 equiv of 3,5-bis-tert-butylsalicylic aldehyde to afford the DIANANE-salen ligand 2 (Schemes 1 and 3). In the course of the crystallization …
The invention provides a preparation met...
The present invention provides compounds...
A simple and efficient procedure to synt...
The stability of 3,5-di-tert-butylsalicy...
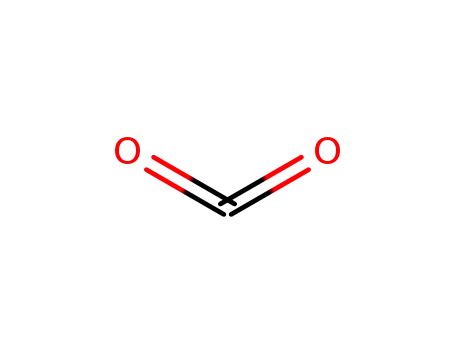
carbon dioxide

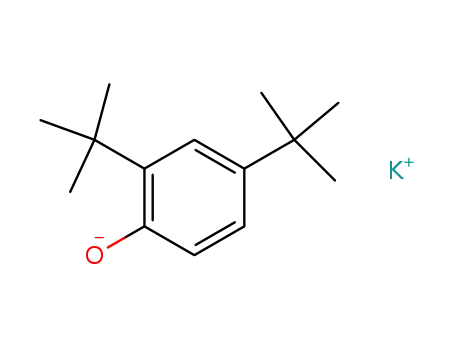
potassium 2,4-di-tert-butylphenolate

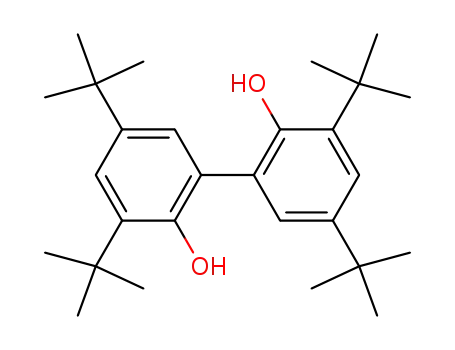
3,3',5,5'-tetra(tert-butyl)biphenyl-2,2'-diol

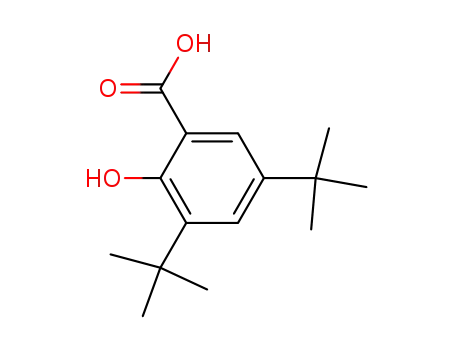
3,5-ditertbutyl salicylic acid
| Conditions | Yield |
|---|---|
|
under 43957.6 - 46543.3 Torr;
|
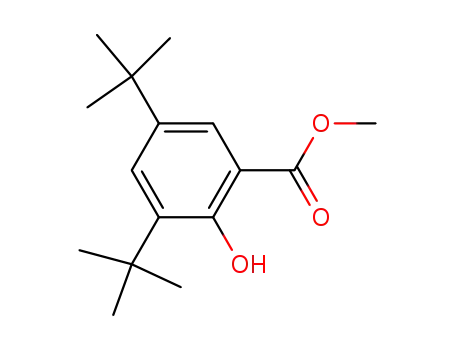
methyl 3,5‐di‐tert-butyl‐2‐hydroxybenzoate


3,5-ditertbutyl salicylic acid
| Conditions | Yield |
|---|---|
|
With water; potassium hydroxide; In methanol; for 1h; Reflux;
|
92% |
|
In methanol; water; for 5h; Heating;
|
80% |
|
With potassium hydroxide; In methanol; for 4h; Heating;
|
75% |
|
With potassium hydroxide; In methanol; water;
|
|
|
With sodium hydroxide; In methanol; at 70 - 90 ℃; for 3h; Reagent/catalyst; Solvent; Temperature;
|
152 g |

carbon dioxide

potassium 2,4-di-tert-butylphenolate

methyl 3,5‐di‐tert-butyl‐2‐hydroxybenzoate
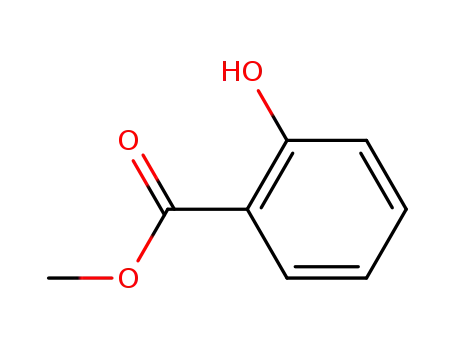
methyl salicylate
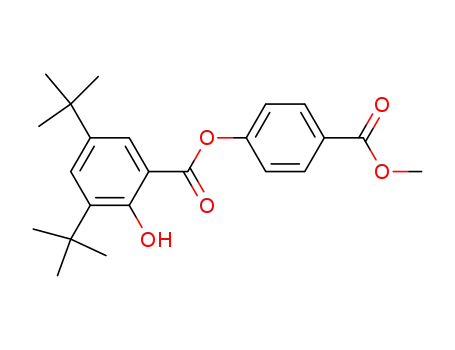
4-carbomethoxyphenyl 3,5-di-t-butylsalicylate
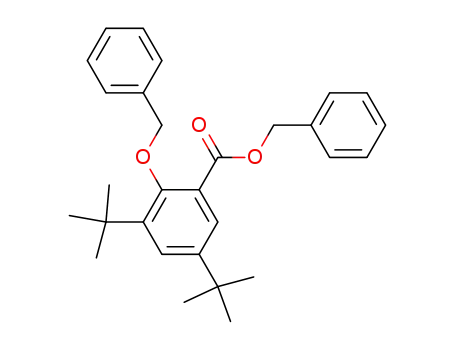
benzyl 2-benzyloxy-3,5-di-tert-butylbenzoate
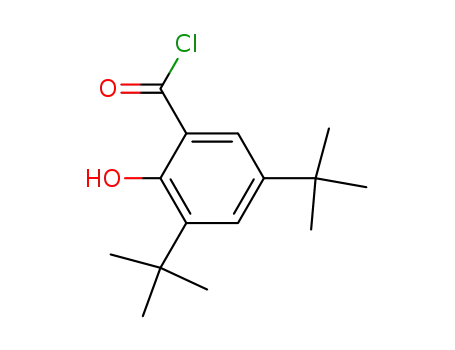
3,5-di-tert-butylsalicylic acid chloride
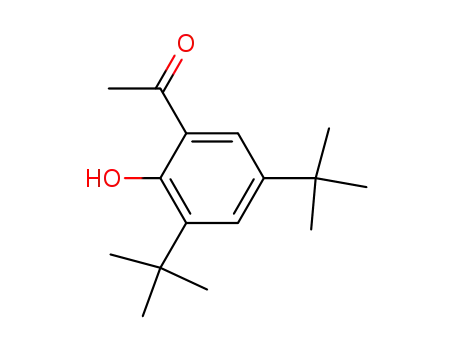
2,4-di-tert-butyl-6-acetylphenol