
Antioxidant BHT 264
CAS:128-37-0
Purity:99%
Contact Now
We will contact you as soon as possible
Your Location:Home >Products >Cosmetic Ingredients >96702-03-3


Product Details
|
Description |
Ectoine is an osmoregulatory compound produced by some bacteria to adapt to high osmotic pressure in the environment. It is structurally similar to ectoine, which is produced by other microorganisms, but different in some aspects. |
|
Osmolytic properties of ectoine |
Ectoine and 5-hydroxyectoine are produced by microorganisms in response to true osmotic stress, and not just in response to increases in the external salinity. In cases where the build-up of ectoine/5-hydroxyectoine pools has been studied in more detail, there is often a linear relationship between the cellular content of these solutes and the external salinity/osmolarity. This finding implies that bacterial cells can perceive incremental increases in the degree of the environmentally imposed osmotic stress, can process this information genetically/physiologically, and can then set its ectoine/5-hydroxyectoine biosynthetic capacity in a finely tuned fashion to relieve the constraints imposed by high-osmolarity on cellular hydration, physiology, and growth. High-osmolarity-dictated increases in the cellular ectoine pools are largely accomplished through osmotically-responsive increases in the transcription of the ectoine/5-hydroxyectoine biosynthetic genes, although there might be post-transcriptional effects as well. Attesting to the role of ectoine as a potent osmostress protectant is the finding that the disruption of the ectABC biosynthetic genes causes osmotic sensitivity and the genetic disruption of the gene (ectD) for the ectoine hydroxylase in Chromohalobacter salexigens impairs the ability to cope effectively with high growth temperature extremes. In microorganisms that are capable of synthesizing both ectoine and 5-hydroxyectoine, a mixture of these two solutes is frequently found. Interestingly, such a 1:1 mixture (0.5 mM each) provided the best salt and heat stress protection to Streptomyces coelicolor when it was added to the growth medium. However, there are also microorganisms that seem to produce almost exclusively 5-hydroxyectoine during osmotic stress and different growth phases of the culture. |
|
existence |
Ectoine is found in high concentrations in halophilic microorganisms and confers resistance towards salt and temperature stress. Ectoine was first identified in the microorganism Ectothiorhodospira halochloris, but has since been found in a wide range of Gram-negative and Gram-positive bacteria. |
|
Uses |
Ectoine is a natural protector that stabilizes proteins and other cell structures and protects the skin from stresses such as UV exposure and dryness. |
|
Definition |
Ectoine is a carboxamidine heterocycle obtained by formal condensation of (2S)-2,4-diaminobutanoic acid with acetic acid. It has a role as an osmolyte. It is a carboxamidine, a member of 1,4,5,6-tetrahydropyrimidines and a monocarboxylic acid. It is a conjugate acid of an ectoinate. It is a tautomer of an ectoine zwitterion. |
|
Biochem/physiol Actions |
Ectoine is an osmoprotectant in a variety of microorganisms, including heterotrophic, halophilic and non-halophilic bacteria, such as Streptomyces and E. coli. This compatible solute is also protective for E. coli during drying and storage. Ecdysone is associated with the stabilization of protein and lipid bilayers. It also regulates the retention of bulking pressure and reduces desiccation stress. It can be used in the treatment of inflammatory diseases such as allergic rhinitis, atopic dermatitis and chronic obstructive pulmonary disease. Ecdysone causes structural changes in DNA in vitro and protects DNA from ionizing radiation (IR). It prevents the formation of insulin amyloid in vitro.Ecdysone is an osmoprotectant in a variety of microorganisms, including heterotrophic, halophilic and non-halophilic bacteria, such as Streptomyces and E. coli. This compatible solute is also protective for E. coli during drying and storage. Ecdysone is associated with the stabilization of protein and lipid bilayers. It also regulates the retention of bulking pressure and reduces desiccation stress. It can be used in the treatment of inflammatory diseases such as allergic rhinitis, atopic dermatitis and chronic obstructive pulmonary disease. Ecdysone causes structural changes in DNA in vitro and protects DNA from ionizing radiation (IR). It prevents the formation of insulin amyloid in vitro. |
InChI:InChI=1/C6H10N2O2/c1-4-7-3-2-5(8-4)6(9)10/h5H,2-3H2,1H3,(H,7,8)(H,9,10)/t5-/m0/s1
Showdomycin is a C-nucleoside bearing an...
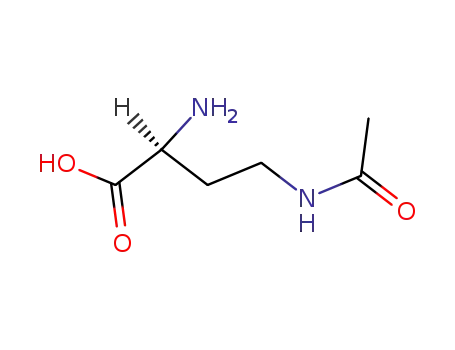
N-γ-acetyl-L-2,4-diaminobutyrate

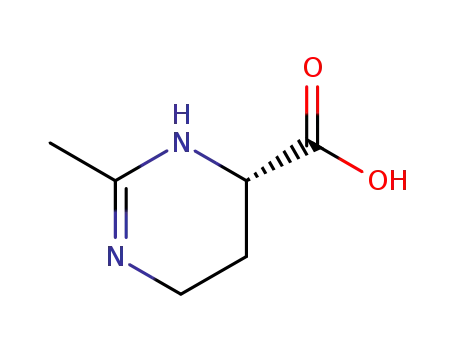
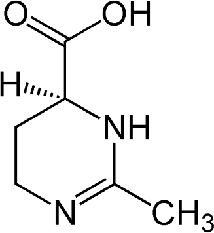
ectoin
| Conditions | Yield |
|---|---|
|
With l-ectoine synthase; Enzymatic reaction;
|

ectoin

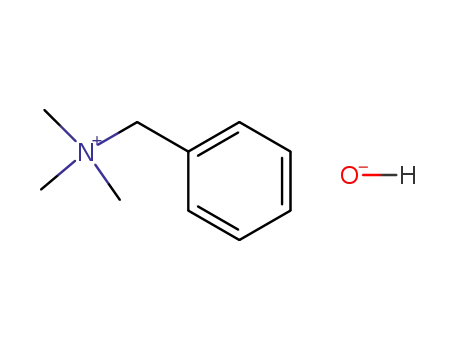
N-benzyl-trimethylammonium hydroxide

benzyltrimethylammonium 2-methyl-3,4,5,6-tetrahydropyrimidine-4-carboxylate
| Conditions | Yield |
|---|---|
|
In water; at 20 - 90 ℃;
|

N-γ-acetyl-L-2,4-diaminobutyrate